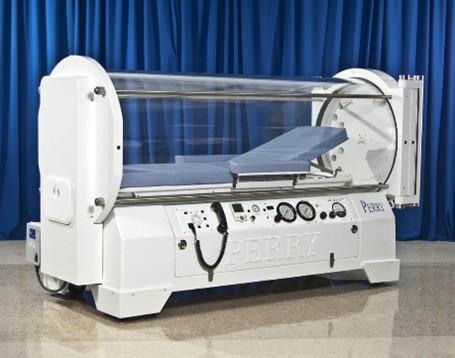
Hyperbaric chambers are pressure vessels designed to provide oxygen therapy for patient care in medical facilities. As hyperbaric chambers are categorized as Pressure Vessels for Human Occupancy (PVHO), they fall under the regulation of the Technical Standards and Safety Authority (TSSA).
The importance of hyperbaric chamber safety was highlighted by an incident in January 2025, when a hyperbaric chamber exploded in Detroit, Michigan. The explosion resulted in the immediate death of a five-year-old child who was undergoing treatment in the pressure vessel.
To ensure the safety of hyperbaric chambers, TSSA conducts regulatory reviews and inspections on the following:
Hyperbaric Chambers
- Register Designs: Hyperbaric chamber designs must be registered with TSSA for review to ensure they comply with adopted safety codes and standards. Applicable codes and standards include CSA B51 Boiler, Pressure Vessel, and Pressure Piping Code, and ASME PVHO-1 Safety Standard for Pressure Vessels for Human Occupancy. Each hyperbaric chamber must have a nameplate stamped with a Canadian Registration Number (CRN).
- Manufacturing Quality Standards: TSSA conducts manufacturing inspections of hyperbaric chambers manufactured in Ontario. Ontario manufacturers are audited by TSSA and issued a certificate indicating their quality systems and process are compliant with the codes and standards.
- Undergo a First Installation Inspection: Before a hyperbaric chamber can be used for patient care in Ontario, it must undergo a first installation inspection by TSSA to ensure the installation is compliant. If a hyperbaric chamber passes the first installation inspection, a unique identification number is assigned, and a Certificate of Inspection (COI) issued allowing the hyperbaric chamber to be put into service.
- Display COIs Prominently: COIs must be posted on or near hyperbaric chambers, or a notification in lieu of posting the COI which clearly indicates the alternate storage location for the COIs. COIs indicate that a device has been inspected and can be safely operated. As such, they shall be available for viewing at all times.
- Periodic Inspections for Pressure Vessels Fitted with Quick Openings: PVHOs that can open and close quickly for repeated internal access are classified as Pressure Vessels Fitted with Quick Opening Doors. These vessels undergo an annual periodic inspection by either TSSA or the insurance company’s inspector to assess their continued safe operation and to renew their COI.
Medical Gas Piping
- Cryogenic Pressure Vessels are also Regulated: Cryogenic pressure vessels that supply oxygen to hyperbaric chambers also require a CRN and a COI.
- Register Designs of Medical Gas Piping: TSSA regulates the design and installation of medical gas piping. Since piping designs are unique to each location, they must be registered and reviewed by TSSA engineers.
- Installation by Certified Contractors: Only contractors with a valid Certificate of Authorization (COA) from TSSA are allowed to install and commission medical piping systems. TSSA regularly inspects and audits contractors with a COA.
- Post-Installation Inspection by Certified Inspectors: All registered medical gas piping is inspected by certified inspectors during the commissioning process.
Hyperbaric chambers must be built using designs and systems compliant with well-established codes and standards and manufactured in facilities with quality control systems. Additionally, hyperbaric chambers must be installed by a competent contractor and inspected periodically to ensure they are maintained and can operate safely.
For more information on registering hyperbaric chamber designs, please send an email to bpv_inquiries@tssa.org.